Are you ready to explore how we can help you?
Our world-class experts are available to help find answers to your toughest questions.

From UV to NIR and longer wavelengths, absorbance measurements provide valuable information about the chemical composition of materials in all states of matter.
Light incident on a sample can be transmitted, absorbed or scattered. Transmission is the light that passes through the sample; light that encounters a molecule can be absorbed or scattered. Absorbance can be used as a qualitative tool to identify substances, or as a quantitative tool to measure the concentration of a molecule in solution.

In this video, we demonstrate a basic absorbance measurement using Ocean Optics products. We also explain OceanView software procedures and share tips to ensure the best results.
Learn more about continuous, online monitoring of proteins using UV absorbance. See how an Ocean HDX spectrometer captures real-time protein concentration values, and then converts the data into meaningful answers.
When configuring a setup for absorbance measurements, your first question should be what you hope to accomplish. Is this a system for general lab use? For teaching lab purposes? For monitoring a manufacturing or testing process?
With your outcome in mind, here’s some good news: Ocean Optics has the tools and expertise to help you configure spectral systems for hundreds of different absorbance applications.
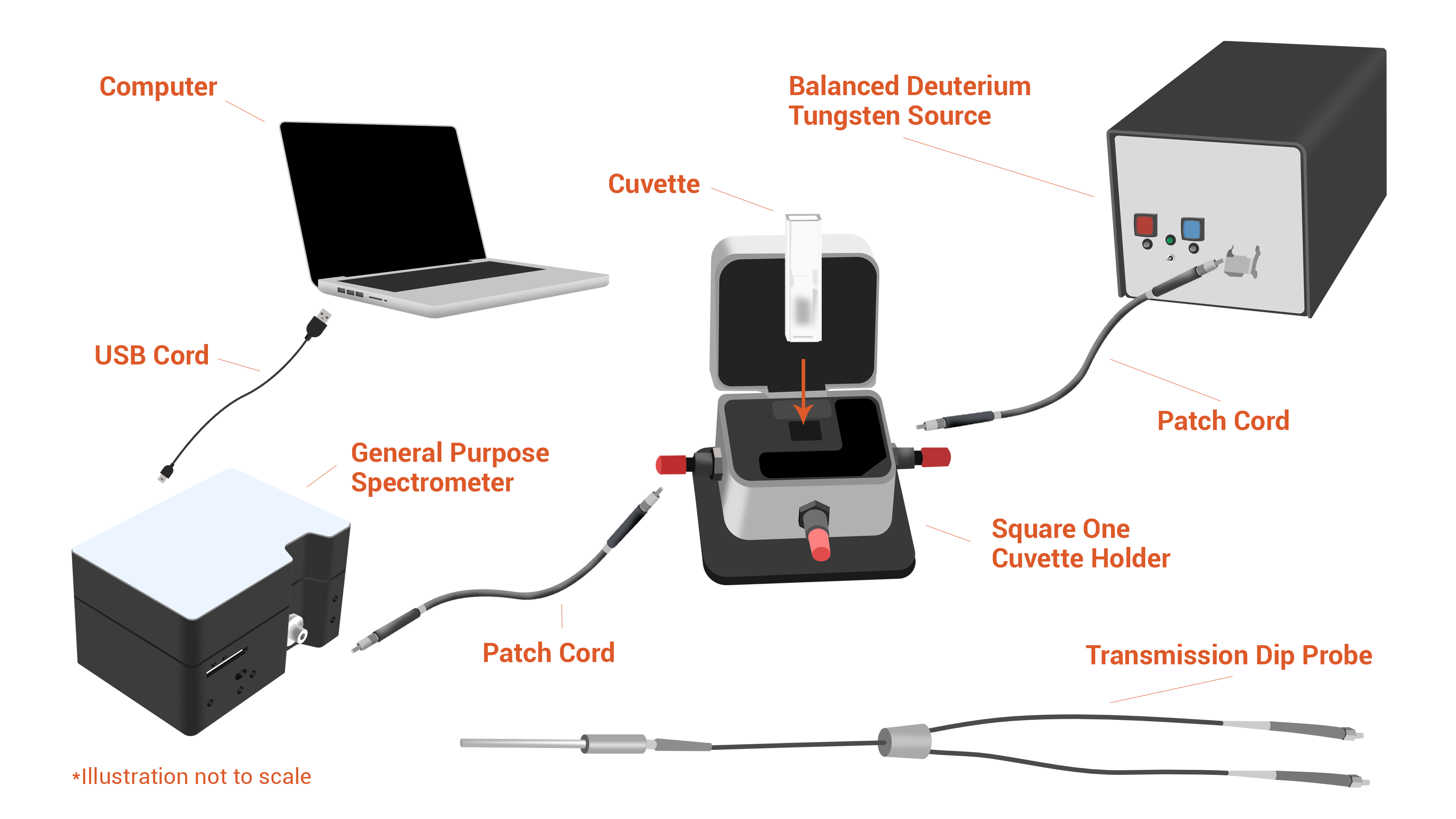
Consider the setup in the illustration. Configuring a system for general-purpose absorbance begins with a spectrometer like the Ocean SR series or the Ocean HDX, which both cover the UV-Vis range, or NIRQuest+ for the NIR. Add a light source – we’re showing a deuterium-halogen, which provides output across the UV, Vis and NIR – and your halfway there.
To complete the system, you’ll need a sampling setup. A cuvette holder for 1 cm pathlength cuvettes is a great start, although for in situ measurements you’ll want a transmission dip probe. With a cuvette holder, you’ll also need an optical fiber to illuminate the sample cell and another fiber to return the signal to the spectrometer.
You may also consider adding an absorbance standard to verify the photometric accuracy of your measurements.
The finishing touch? Set up your experiment in OceanView operating software using our simple, step-by-step Absorbance Wizard.
To observe the UV response and absorbance linearity of the SR6 spectrometer, we measured the absorbance of various concentration levels of DNA samples in water and of NIST-traceable potassium dichromate standards.
With excellent SNR performance and high-speed spectral acquisition, the Ocean SR2 spectrometer is ideal for absorbance of optical filters and potassium dichromate standards.
In this application note, we use an Ocean HDX spectrometer to generate a standard curve for bovine serum albumin (BSA).
This 1 cm pathlength cuvette holder is ideal for accurate, repeatable absorbance and fluorescence measurements. Square One provides a snug, reliable fit for cuvettes and filters. Download Product Sheet to learn more!

Our world-class experts are available to help find answers to your toughest questions.