Using a variety of spectroscopy and sensing techniques, we help you harness the power of light to return precise, actionable information about the world around you. The power of spectroscopy lies in its ability to probe materials and environments in a variety of different ways. Here’s what we’ve learned about those techniques, and what you need to know about applying them using Ocean Optics products.
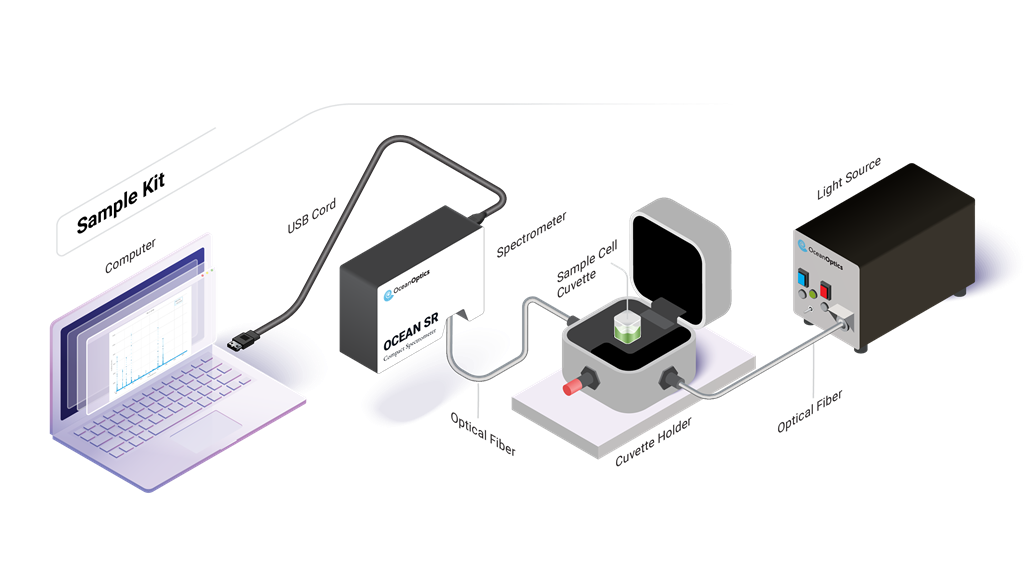
Perform a wide range of spectroscopic experiments, making it a valuable tool for learning and research. Spectroscopy Kits provide users with versatile tools for utilizing absorbance, color, reflectance, and other techniques.
From UV to NIR wavelengths, absorbance measurements provide information about the chemical composition of materials in all states of matter. Learn how absorbance can be used to identify substances or measure the concentration of a molecule.
In simplest terms, color is one of the ways humans perceive light. Color reflected from, or emitted by samples can be measured using basic color meters or more robust spectral devices for detailed analysis of different color properties.
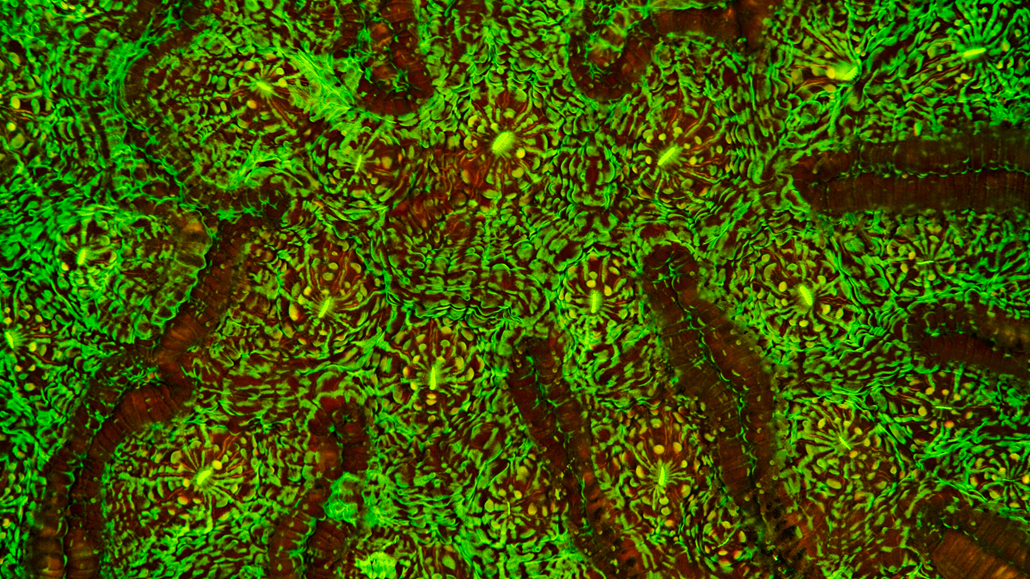
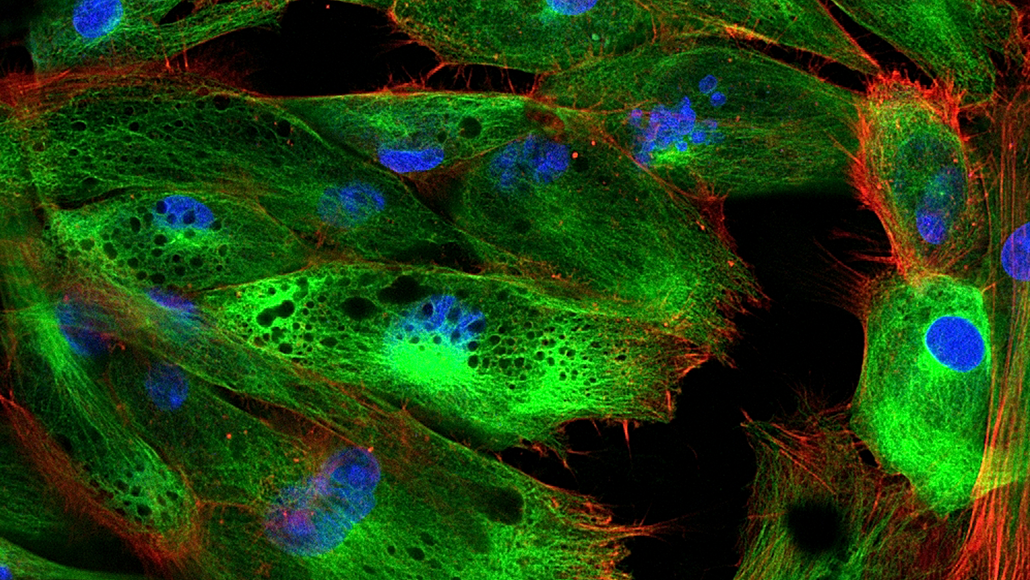
Fluorescence is the absorption and emission of light of two different frequencies. Typically, a lower wavelength of incident light is absorbed from one direction, and a higher wavelength of light is emitted in all directions.
Irradiance is the amount of energy emitted at each wavelength from a radiant sample such as an LED, a laser or the sun. From that data, more specific intensity values can be calculated.
Multispectral imaging (MSI) involves capturing images of a scene or object over multiple discrete wavelength bands and extracting spectral content from that data.
Optical oxygen sensors comprise an indicator fluorophore in a sol-gel host matrix that’s applied to an adhesive patch or fiber tip. The indicator changes optical properties in response to analytes in its environment and a fluorometer measures the response.
Optical pH sensors comprise a pH indicator dye in a matrix applied to a patch or cuvette, which changes color in response to analytes in its environment. A spectrometer measures the colorimetric (absorbance) response.
Raman spectroscopy uses scattering of laser light to probe molecular structure. Of every million photons scattered, a single photon will interact with the vibrational states of a sample molecule and emit light of a different wavelength.
Reflectance is light incident on the surface of a material that is reflected at an interface. Light not reflected from the sample is absorbed, scattered or transmitted.
Transmittance is light incident on a sample that transmits through it. If the sample has low scatter (as with a clean, dilute solution), almost all the light not absorbed will be transmitted.

Are you ready to explore how we can help you?
Our world-class experts are available to help find answers to your toughest questions.